|
Size: 2188
Comment:
|
Size: 2233
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 19: | Line 19: |
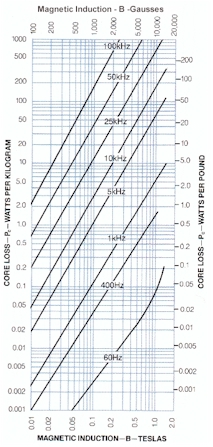
| || [[attachment:2605SA1_coreloss.jpg | {{attachment:2605SA1_coreloss.jpg}}]] || Core loss appears proportional to (frequency × flux )^1.8^ . ]] || | || [[attachment:2605SA1_coreloss.jpg | {{attachment:2605SA1_coreloss.jpg}}]] || Core loss appears proportional to <<BR>><<BR>> (frequency × flux )^1.8^ .<<BR>><<BR>> Figure from datasheet. || |
Rotor Lamination
Laminated launch loop rotors will have good magnetic properties and will rapidly disperse and oxidize in the atmosphere after a rotor-release catastrophe. Worst case, thin flakes survive and cut.
Metglas 2605SA1 looks good, higher temperature than 2605HB1M. datasheet downloaded 2017/02/11
Metglas 2605SA1 |
||||
Curie temperature |
395C / 668K |
|
saturation induction |
1.56 Tesla |
thickness |
23 μm |
|
density |
7.18 g/cm³ |
Thermal Expansion |
7.6 ppm/°C |
|
Iron vaporization temp |
3140 K |
Tensile Strength |
1 GPa |
|
Elastic Modulus |
100 GPa |
Iron |
85 to 95% |
|
2.5g/m³ |
|
Silicon |
5 to 10% |
|
3.0g/m³ |
|
Boron |
1 to 5% |
|
2,0g/m³ |
|
resistivity |
1.3 μΩ-m |
|
|
|
60 Hz and 1.4 T |
|
Induction at 80 A/m |
≥1.35 T |
|
Core Loss |
≤0.17 W/kg |
|
Exciting Apparent Power* |
1.1 (VA/kg) |
Failure and oxidation
Iron has a specific heat of 25.1 J/(mol·K), vaporizes at 3140K, and has a heat of vaporization of 340 kJ/mol . Naively, to go from 400K to vaporization requires 410 kJ/mol or 16 MJ/kg . The rotor moves at 14 km/s, the kinetic energy is 98 MJ/kg, so there is more than enough energy to vaporize the rotor foil and ignite it. Further study and experimentation needed; perhaps most of the energy will end up in the surrounding air.