|
Size: 899
Comment:
|
← Revision 11 as of 2021-01-28 08:12:27 ⇥
Size: 1234
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 5: | Line 5: |
| MoreLater |
|
| Line 7: | Line 9: |
| [[ attachment:cryoaluminum1.png | {{ attachment:cryoaluminum1.png | | }} ]] | [[ attachment:cryoaluminum2.png | {{ attachment:cryoaluminum2.png | | }} ]] |
| Line 9: | Line 11: |
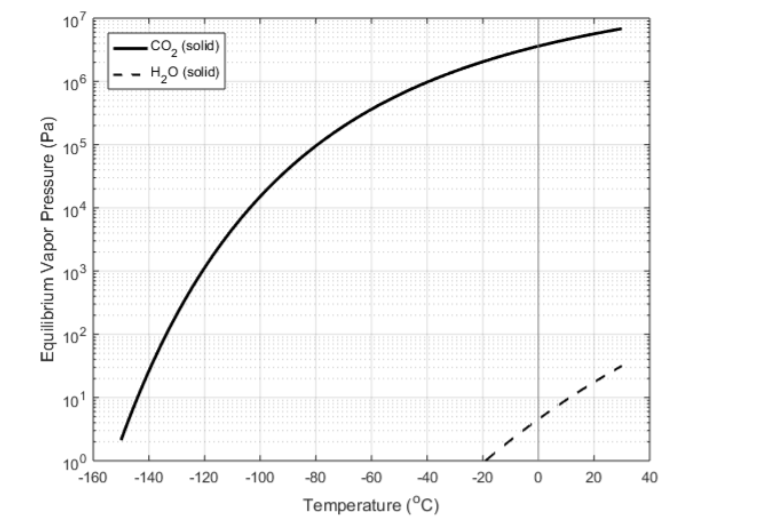
| LN2 seems a little too cold to be an optimum "contact" coolant. It has a heat capacity of 1040 Joules per kilogram-Kelvin, slightly better than liquid water around room temperature. | LN2 seems a little too cold to be an optimum "contact" coolant. It has a heat capacity of 1040 Joules per kilogram-Kelvin, slightly better than liquid water around room temperature, and a latent heat of evaporation of 199 kJ/kg. So how about dry ice, frozen CO2? [[ attachment:CO2ice.png | {{ attachment:CO2ice.png | | }} ]] The [[ https://webbook.nist.gov/cgi/inchi?ID=C124389&Mask=4 | enthalpy of sublimation of CO2 ]] is 26 kJ/mole at 167K (-110C ). A mole is 0.044 kg, so that is 600 kJ/kg . |
Cold Launch Sled
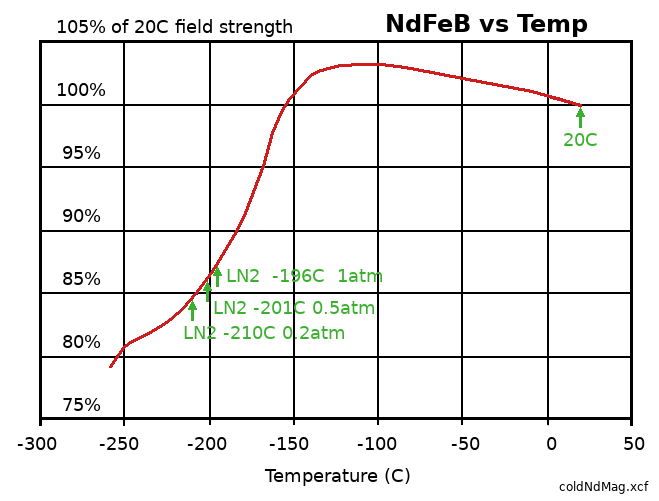
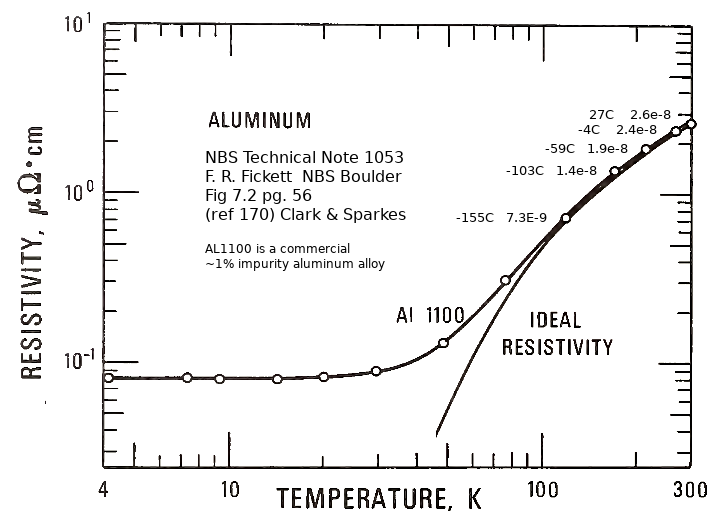
Neodymium (NdFeB) magnet remanence (magnetization, in Teslas) decreases with temperature above 20C. Remanence is maximum, 3% higher than 20C "room temperature", at -110C. Remanence falls off below that temperature, reduced to 100% again at -155C, and reduced to 87% at -196C (liquid nitrogen boiloff temperature at one atmosphere pressure). Chilling the sled magnets below 150C before launch will maximize their remanence later, during the middle of the launch run, and also reduce the resistivity of the aluminum eddy current shield.
LN2 seems a little too cold to be an optimum "contact" coolant. It has a heat capacity of 1040 Joules per kilogram-Kelvin, slightly better than liquid water around room temperature, and a latent heat of evaporation of 199 kJ/kg. So how about dry ice, frozen CO2?
The enthalpy of sublimation of CO2 is 26 kJ/mole at 167K (-110C ). A mole is 0.044 kg, so that is 600 kJ/kg .